- Home
- Company
- Manufacturing Facilities
- Anseong Plant
- Introduction
Anseong Plant
안성공장 외부 이미지
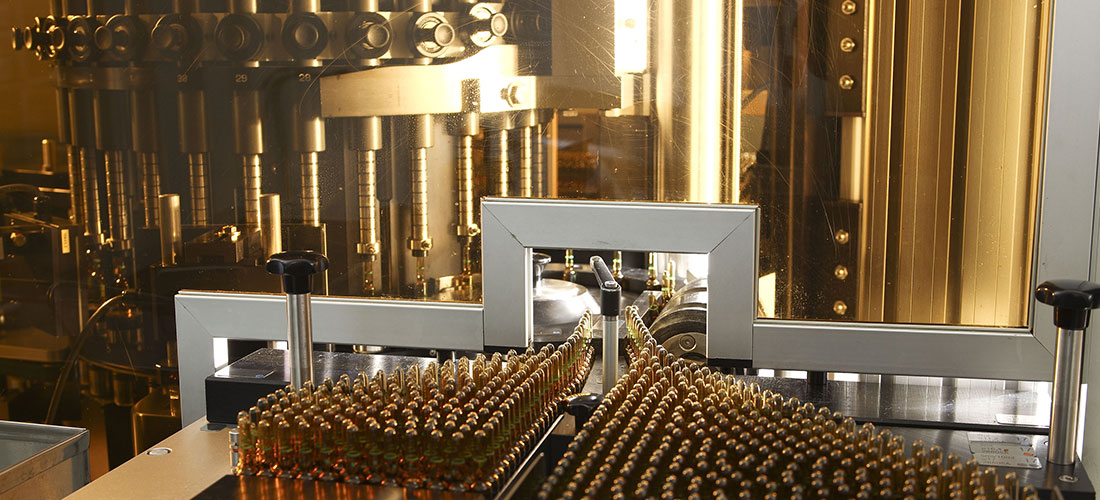
Ildong Pharmaceutical’s manufacturing facility in Anseong was the facility in the country to acquire the KGMP certification for all its processes and currently combines the latest production equipment with strict GMP standards to manufacture drug products of the highest quality.
The facility consists of separate cephalosporin antibiotics and cytotoxic anticancer agents manufacturing buildings that are compliant with GMP standards and utilizes the one-line system, the restricted access barrier system, the building management system, the automated warehouse system, and other advanced manufacturing solutions to prevent contamination at its roots, create the most optimal environment for manufacturing pharmaceuticals, and ensure the manufacture of defect-free drug products. The cephalosporin antibiotics building and cytotoxic anticancer agents building are capable of producing approximately ₩150 billion and ₩50 billion’s worth of products each year, respectively, and constitute key elements of Ildong Pharmaceutical’s global expansion strategy.
Anseong Facility’s Key Products
- General facility
-
- Solids : Aronamin Gold Tablet, Curan Tablet, Biovita Granule, and others
- Injectables : Ativan Injection, Curan Injection, and others
- Cephalosporin Antibiotics
-
- Solids : Flomox Tablet, Cefaclor Capsule, and others
- Injectables : Flumarin Injection and others
- Cytotoxic anticancer agents
-
- Solids : Xelobig tablet, Temolam capsule, etc.
- Injectables : Flumarin Injection and others
Location
-
Address
25, Gongdan 1-ro, Anseong-si, Gyeonggi-do, Republic of Korea
-
Telephone
+82(31)673-1701
-
Facsimile
+82(31)673-4860
Ildong Pharmaceutical’s manufacturing facility in Anseong combines modern production equipment with strict GMP standards enforced by teams of skilled professionals on per-drug-type and per-process basis to ensure the manufacture of products of the highest quality.
The facility is divided into an injectables building, an internal-solids building, and an administrations and logistics building and operates separate manufacturing areas for products containing lactobacillus bacteria and products containing crude drugs. Furthermore, anterooms have been installed at the entrances to internal-solids preparation, tableting, coating, and filling areas to prevent cross-contamination.
| Building | General facility(Building G) |
|---|---|
| Building Area | 25,101㎡ |
| Completion | 2013 |
| Features |
|
| KGMP Approval | Revised approval in Dec 2013 (injectables and internal solids) |
| MFDS PIC/S GMP Compliance | 31 August 2016 |
Manufacturing Capacity
- Freeze-dried Inejctables
- 18,250,000 vials
- Liquid Injectables
- 73,600,000 vials
- Ampule Injectables
- 94,600,000 ampules
- Tablets
- 11,300,000,000 tablets
- Capsules
- 2,500,000,000 capsules
- Granules
- 660,000 kg
Floor by Floor
| Floor | Injectables Building (G1) | Internal Solids Building (G2) | Administrations & Logistics Building (G3) |
|---|---|---|---|
| 3 | HVAC | HVAC | Quality Management |
| 2 | Injectables Preparation Area Raw Materials Storage | Internal Solids Preparation Area | Office Conference Room |
| 1 | Injectables Packaging | Internal Solids Packaging Raw Materials Storage | Finished Products Storage |
Manufacturing
Ildong Pharmaceutical’s manufacturing facility in Anseong combines advanced and modern manufacturing solutions rivaling those of some of the most recognized names in the pharmaceuticals industry with strict GMP standards implemented per product type and per process by a staff of experts to manufacture pharmaceuticals of the highest quality. The facility also practices production-innovation protocols known as 5S and TPM and is constantly striving to achieve increasingly higher levels of product quality and manufacturing productivity.
Quality Management
From sourcing raw materials to manufacturing and packaging finished products, Ildong Pharmaceutical’s manufacturing facility in Anseong strictly adheres to GMP standards and performs internally developed inspections and tests through the hands of trained and experienced quality-control personnel and using the latest equipment to ensure consistent levels of quality, efficacy, and stability in all its products. The facility’s personnel also include a separate quality-assurance team responsible for validating manufacturing processes and quality tests and for administrating a quality-enhancement system.
- Ildong Quality System
- EU CMP
- KGMP
- ICH Guidelines
- PIC/S Guidelines
Support System & Environmental Management
The facility includes four separate areas for storing raw materials, supplies, and finished products (5,000 cells in total) and utilizes
advanced systems to separate and store the materials, supplies, and products without any cross-contamination.
An advanced system for managing the quality of water used at the
facility has also been incorporated to maintain even higher water quality
than required and prevent chemical byproducts from entering the nearby
waterways or the atmosphere.
Through the use of advanced solutions and a strong sense of
responsibility for the preservation of the environment,
Ildong Pharmaceutical continues to serve as a steward in charge
protecting the environment for future generations.
The cephalosporin antibiotics building is where freeze-dried injectables, powdered injectables, internal solids, capsules, dry syrups, and other types of drugs are manufactured.
The building features two freeze-drying units capable of manufacturing 120,000 vials simultaneously and a manufacturing system approaching full automation, achieving unmatched levels of productivity and quality. The building also features an ERP system, a BMS (Building Management System), and an automated warehouse to achieve the most optimal environment for manufacturing pharmaceuticals.
| Building | Cephalosporin antibiotics facility(Building C) |
|---|---|
| Building Area | 7,587㎡ |
| Machinery Installation | December 2009 |
| Completion | November 2009 |
| Features |
|
| KGMP Approval | Revised approval in July 2015 (injectables and internal solids) |
| MFDS PIC/S GMP Compliance | 14 July 2015 |
Manufacturing Capacity
- Freeze-dried Injectables
- 11,000,000 vials
- Powdered Injectables
- 37,000,000 vials
- Tablets
- 500,000,000 tablets
- Capsules
- 660,000,000 capsules
- Dry Syrups
- 650,000 kg
Floor by Floor
| Floor | Category | Formulation Type | Area (㎡) |
|---|---|---|---|
| Rooftop | HVAC | 874 | |
| 3 | Solids | Tablets Capsules Dry Syrups | 1,773 |
| 2 | QC/QA | 818 | |
| 1 | Automated Warehouse Cafeteria | Freeze-dried Injectables Powdered Injectables | 2,312 |
| Basement | Water Reservoir, Transformers, Generators, and Water-Treatment System | 1,902 | |
Features
- Cephalosporin Injectables
-
- Complete one-line automation from vial washing to packaging
- Contamination-proof filling, freeze-drying, and capping via RABS
- Auto-loading of vials onto freeze-driers after filling
- Vial exterior cleaning to eliminate contamination during the manufacture process
- Cephalosporin Solids
-
- Systems for manufacturing tablets, capsules, and dry syrups
- Weighing cabinets to prevent cross-contamination during weighing
- IBCs and automated washers to prevent cross-contamination
- Use of air-jet mills for greater manufacturing efficiency and throughput
The anticancer drugs building at Ildong Pharmaceutical’s manufacturing facility in Anseong is the country’s first independent cytotoxic chemotherapeutic agents facility capable of producing both injectables and solids.
The building features fluid-bed granulators capable of mixing, combining, assembling, and drying in a single line, is designed to fully contain cytotoxic substances within the facility, and utilizes airtight bins throughout the entire manufacturing process to prevent airborne contamination.
| Building | Cytotoxic anticancer agents facility(Building A) |
|---|---|
| Building Area | 2,344㎡ |
| Machinery Installation | December 2009 |
| Completion | November 2009 |
| Features | Manufacture of cytotoxic chemotherapeutical agents in internal solid and injectable formulations |
| KGMP Approval | September 2010 (injectables) September 2011 (internal solids) |
| MFDS PIC/S GMP Compliance | 14 July 2015 |
Manufacturing Capacity
- Freeze-dried Injectables
- 550,000 vials
- Liquid Injectables
- 30,000,000 vials
- Tablets
- 100,000,000 tablets
- Capsules
- 300,000,000 capsules
Floor by Floor
| Floor | Category | Formulation Type | Area (㎡) |
|---|---|---|---|
| Rooftop | HVAC | 390 | |
| 2 | Solids | Tablets and Capsules | 314 |
| 1 | Injectables Warehouse | Freeze-dried Injectables Liquid Injectables | 1,378 |
| Basement | Water-Treatment System | 87 | |
Features
- Personnel Protection from Cytotoxic Substances
-
- Dust-Collection System
- Enclosed Weighing Station
- Protective Headgear and Clothing
- State-of-Art Manufacturing Equipment
-
- Fully Automated Vial-Production Line
- Aseptic Clean Carts
- Wet Granulator